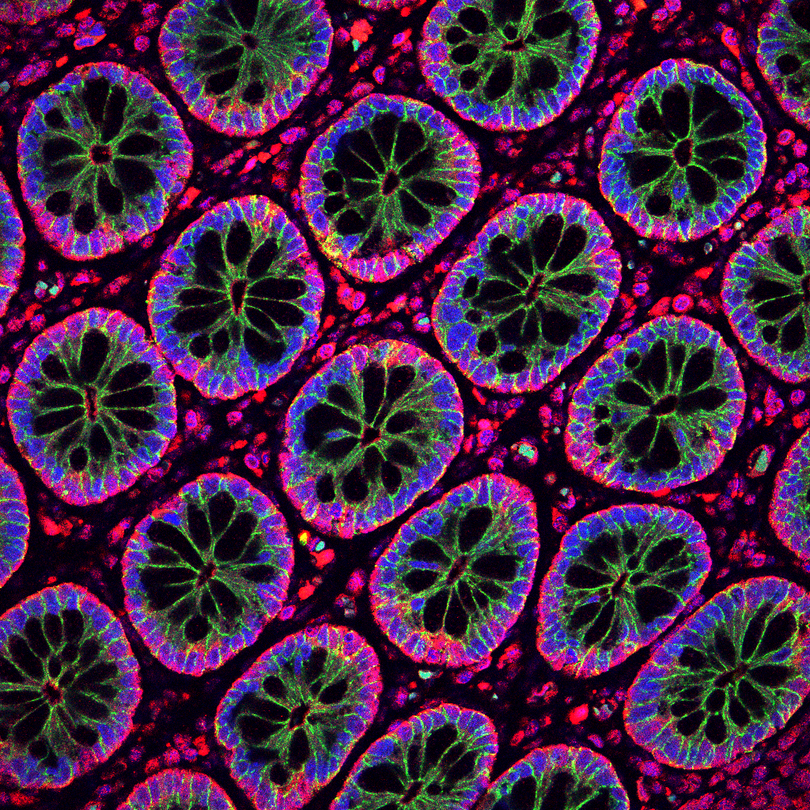
Cell-cell junctions and RNA-associated complexes have an intimate affair...
The adherens junctions are cadherin-dependent, cell-cell adhesion structures critical for the development and maintenance of tissue architecture. Disruption of adherens junctions results in loss of tissue integrity and has been associated with numerous diseases, including cancer. Our recent work investigating the mechanisms through which cadherin junctions influence cell behavior led us to the discovery of a novel interaction with the RNAi machinery. We have shown that epithelial cadherin complexes recruit the microprocessor and RNA-induced silencing (RISC) complexes, as well as specific sets of miRNAs and mRNAs, through the E-cadherin - p120 catenin - associated protein called PLEKHA7. By recruiting RNAi, cadherin junctions of well-differentiated epithelial cells regulate processing and activity of miRNAs to suppress expression of promoters of cellular transformation. This was a breakthrough finding that challenged the prevailing dogma that the microprocessor localizes and functions solely in the cell nucleus and the RISC in the cytoplasm. Current findings in our lab portray an extensive crosstalk of epithelial cadherin junctions with a variety of coding, non-coding, and small RNA species, as well as with numerous RNA-binding proteins, revealing a previously unappreciated epicenter of RNA regulation in the cell.
...that impacts cell behavior...
Our work has demonstrated that disruption of the cadherin-associated RNAi complex, through PLEKHA7 loss, results in compromised epithelial integrity, decreased miRNA levels and activity, upregulation of a number of growth-promoting and oncogenic mRNAs, and increased anchorage-independent growth, in vitro and in vivo, which is a hallmark of pro-tumorigenic cell transformation. We are also seeing widespread disruption of this mechanism in tissues from epithelial tumors, such as from patients with colorectal cancer. Together, these findings portray a key role of this mechanism in the maintenance of the normal epithelial phenotype and outline its putative function as a novel tumor suppressor. These findings may have important implications, both in our understanding of the mechanisms of cellular homeostasis, as well as in identifying novel causes of disease progression, such as in cancer, eventually leading into the development of novel therapeutics.
...and opens numerous directions of investigation
Our research is unique in bringing together the fields of cell-cell adhesion and RNA biology, introducing a novel area of investigation. It also poses numerous intriguing questions: why and how do RNA-related complexes recruited to the adherens junctions in the first place and why is this critical for their function? which is the full spectrum of the RNAs and proteins involved and which ones are eventually the most critical for regulation of cell behavior? how is the functionality of the mechanism fine-tuned? how widespread is the presence of tis mechanism at adherens junctions: is it epithelial-specific, organ-specific, species-specific? what is eventually the role of this mechanism in normal cell physiology and homeostasis? at what level is disruption of this mechanism implicated in disease, particularly in tumorigenesis? can we exploit it for therapeutic intervention? By employing cutting-edge approaches, including super-resolution microscopy, CRISPR/Cas9-generated in vitro and in vivo models, 3D cultures, and next generation sequencing, we are systematically addressing these questions in our lab, through several ongoing projects.